c2h5oh lewis structure|C2H5OH (Ethanol) Lewis Structure in 6 Steps : Tagatay Learn how to draw the Lewis structure of ethanol, a colorless liquid with a distinct odor and a pungent taste. Find out the hybridization, molecular geometry, and pol. Wir kochen frisch, denken modern, verhalten uns lässig und lieben die Gastfreundschaft. Die Siebzehn, Bar & Restaurant in Wertingen.
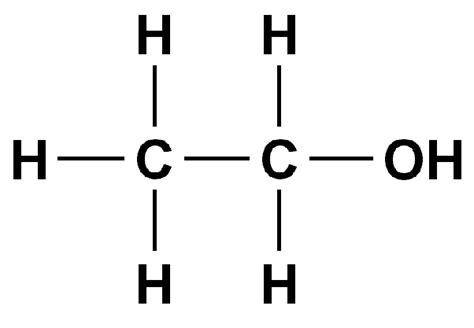
c2h5oh lewis structure,Learn how to draw the Lewis dot structure for ethanol (ethyl alcohol) with 20 valence electrons. Follow the steps to distribute the electrons around the central atom and complete the octets on the outer atoms.Learn how to draw the Lewis structure of ethanol, a colorless liquid with a distinct odor and a pungent taste. Find out the hybridization, molecular geometry, and pol.
Learn how to draw the Lewis structure of ethanol (C2H5OH) and understand its properties, shape and molecular geometry. Find out the number of valence electrons, hybridization, bond angles and lone pairs . Learn how to draw the lewis dot structure of C2H5OH (ethanol) in 6 simple steps with images and examples. Check the stability of the lewis structure by calculating .
c2h5oh lewis structure Hello Guys!In this video, we will determine the Lewis Structure of Ethanol. It has a chemical formula of C2H5OH. To find out its Lewis Structure, we first ca. The Lewis dot structure of ethanol (C2H5OH) shows that the two carbon atoms are bonded together, with one carbon atom also bonded to an oxygen atom, .
Lewis Structure for C2H5OH: https://yout. An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles. Learn how to draw the lewis structure of C2H5OH (ethanol) with 20 valence electrons using 6 steps. Find the valence electrons, select the central atom, connect the atoms, make the outer atoms stable, .

To construct the Lewis structure, we start by counting the valence electrons. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1. In . Ethanol Lewis structure. CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom. In the . An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles. Lewis Structure for C2H5OH: https://yout. Lewis Structure of C2H5OH (Ethanol): Explained. Aspect Explanation Illustration; Valence Electrons: – 2 C atoms (4 e/atom) + 6 H atoms (1 e/atom) + 1 O atom (6 e/atom) = 20 total electrons. – Count valence electrons for each element based on their periodic group. Central Atom: The Lewis structure of ethanol (C 2 H 5 OH) consists of three different elemental atoms, i.e., two carbon (C) atoms, six hydrogen (H) atoms and one atom of oxygen (O).. There are two C-atoms, single . La structure de Lewis C2H5OH (éthanol) a des atomes de carbone (C) au centre qui sont entourés d’atomes d’hydrogène (H) et d’un groupe OH. Il existe cinq liaisons CH, une liaison OH et une liaison CO. Il y a 2 paires libres sur l’atome d’oxygène (O).
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single .Throughout the course, it will be helpful to convert compounds into different structural formulas (Kekule (Lewis Structures), Bond-line, and Condensed) depending on the type of question that is asked. Standardized exams frequently include a high percentage of condensed formulas because it is easier and cheaper to type letters and numbers than .Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.c2h5oh lewis structure C2H5OH (Ethanol) Lewis Structure in 6 Steps A step-by-step explanation of how to draw the CH3CH2OH (C2H5OH) Lewis Dot Structure.FFor the CH3CH2OH structure use the periodic table to find the total numb. Step 2: Add up the valence electrons for each atom in the molecule. For example, H 2 O 2 H: 2 x 1 electron = 2 electrons. 1 O: 1 x 6 electrons = 6 electrons-----Total: 8 electrons. Step 3: (Octet Rule) All covalent bonds are shown by two shared electrons. Place a pair of electrons between two elements that are connected to each other with a . Learn to determine if C2H5OH (Ethanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.
1. Write the condensed formula for each Lewis structure. A. B. 2. Draw a line-angle structure for the compound CH 3 CH 2 CH(CH 3)CH 2 CH 2 CH 3. 3. Give the condensed formula for the compound represented by this line-angle structure: 4. Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds .
Thus far, we have discussed the Lewis structure of atoms and ionic compounds. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions.For example, when two chlorine atoms form a chlorine molecule, they share one pair of .
Ethanol (C2H5OH) is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 2 steps! Step #1: Draw the lewis structure. Here is a skeleton of C2H5OH lewis structure and it contains C-H bonds, C-O bond, C-C bond and O-H bond.Draw a Lewis structure for ethanol (C 2 H 5 OH), then answer the following questions about the structure: Number of valence electrons: Number of bonding electrons: Number of non-bonding electrons: Tries 4/99 Previous Tries Identify the fault, if any, in each of the following Lewis structures for ethanol.If more than one answer appl A Incorrect: Wrong .

For the C2H5 + structure use the periodic table to find the total number of valence electrons for the C2H5 + molecule. Once we know how many valence electron.
C2H5OH (Ethanol) Lewis Structure in 6 Steps It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds. This suggests the best Lewis structure has three B–F single bonds and an electron deficient boron.
Compute formal charges for atoms in any Lewis structure; Use formal charges to identify the most reasonable Lewis structure for a given molecule; Identify the oxidation states of atoms in Lewis structures; 3.3.0: Bond Types. 3.3.0.0: Bond Types (Problems) 3.3.1: Lewis Dot Diagrams.An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group.Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in .
c2h5oh lewis structure|C2H5OH (Ethanol) Lewis Structure in 6 Steps
PH0 · Understanding the Lewis Structure of C2H5OH (Ethanol)
PH1 · Lewis Structure of C2H5OH (Ethanol) (With 6 Simple Steps!)
PH2 · How to Draw the Lewis Dot Structure for C2H5OH: Ethanol
PH3 · Ethanol Lewis structure
PH4 · Ethanol Lewis Dot Structure: Drawing And Detailed Explanations
PH5 · C2H5OH Lewis Structure, Molecular Geometry, Bond
PH6 · C2H5OH Lewis Structure, Molecular Geometry
PH7 · C2H5OH Lewis Structure (Ethanol)
PH8 · C2H5OH (Ethanol): Molecular Geometry and Bond Angles
PH9 · C2H5OH (Ethanol) Lewis Structure in 6 Steps